Hydrothermal Processes
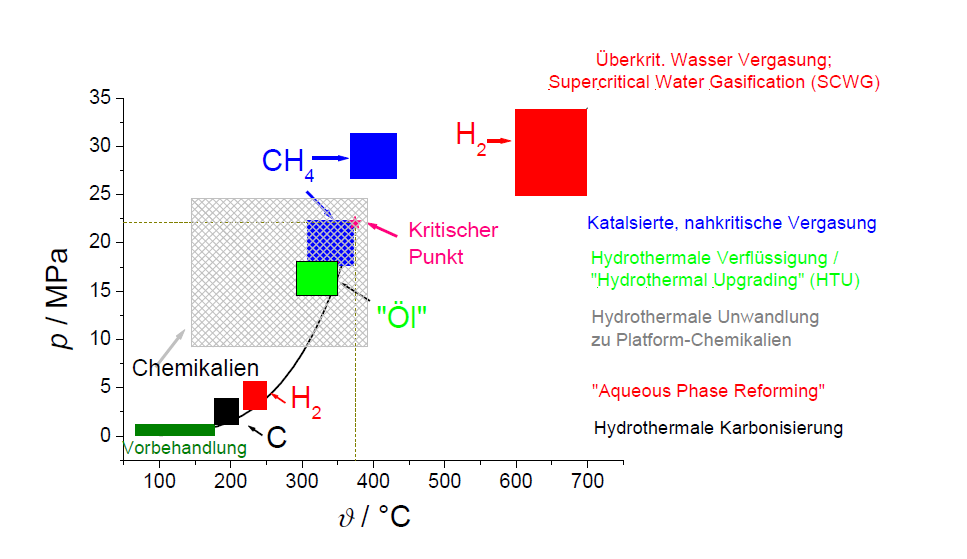
A process in water at raised temperatures and pressures is called a hydrothermal process. The concept comes originally from the geology. The picture on the left shows a simplified overview about different hydrothermal processes and includes the vapour pressure curve of the water. The higher the required temperature for a certain process is the higher the pressure has to be in order to avoid water evaporating. Even if the biomass should be converted biochemically, biomass and its chemical lysis takes places first in a hydrothermal way. The best known process of this kind is the so-called "vapour explosion" („Steam Explosion“) triggered by a sudden reduction of the pressure with the result, that stored water evaporates and the structures of the biomass breakes up. The production of carbon-rich solids is successful in the range of 200°C. This procedure is called "Hydrothermal Carbonisation". At higher temperatures, there is known a method for the production of hydrogen by precious metal catalysts ("Aqueous Phase Reforming"). At approx. 300-350°C biomass can be converted into a high-viscosity tar which has a higher heating value and a higher viscosity as well as a lower water content than liquefaction products from "dry" processes. In a wide range of temperature single-substance syntheses are also known e.g. furfurale erythrose, glycol aldehyde and milk acid made of carbohydrates. From these substances other basis chemicals and finally e.g. plastics are produceable. Near the critical point (371°C, 221 bar) biomass can be converted into methane and carbon dioxide in the presence of precious metal catalysts ("Catalysed Close-critical Biomass Gasification"). At higher temperatures of more than 600°C hydrogen and carbon dioxide are the predominating products ("Overcritical Biomass Gasification"). Typically for hydrothermal processes are - in comparison to "dry ones" developed from the coal conversion - the following process parameters:
- Lower temperature
- Higher pressure
- Higher yields of the requested products e. g. coal
- Wet biomass can be converted directly without any kind of previous drying procedures
Literature
- A. Sinag, A. Kruse, and P. Maniam,
Hydrothermal conversion of biomass and different model compounds,
The Journal of Supercritical Fluids 71 (2012) 80-85. - A. Sinag, T. Yumak, V. Balci, and A. Kruse,
Catalytic hydrothermal conversion of cellulose over SnO2 and ZnO nanoparticle catalysts,
Journal of Supercritical Fluids 56 (2011) 179-185 - A. Kruse,
Supercritical water gasification,
Biofuels, Bioproducts and Biorefining 2 (2008) 415-437. - A. Kruse and H. Vogel,
Heterogeneous Catalysis in Supercritical Media:
2. Near-Critical and Supercritical Water, Chemical Engineering & Technology 31 (2008) 1241-1245. - A. Kruse and E. Dinjus,
Hot compressed water as reaction medium and reactant: Properties and synthesis reactions,
The Journal of Supercritical Fluids 39 (2007) 362-380. - A. Kruse and E. Dinjus,
Hot compressed water as reaction medium and reactant:
2. Degradation reactions, The Journal of Supercritical Fluids 41 (2007) 361-379.