Hydrothermal and Solvolytic Splitting of Lignin
The wood pulping process for the cellulose production creates every year 70 million t of lignin in various and modified forms of waste liqour. The solid parts of the waste liqour consist one third up to half lignin components. The remaining part of the waste liqour consists wood components, coal hydrates or the by-products of coal hydrates as well as chemicals from the wood pulping process which are won back mainly after the combustion of the organic components. Lignin is a natural, three-dimensional branched macromolecule of keto and ether bondings connected with phenylpropylderivates. Hence lignin - in comparison to cellulose and hemicellulose - can act as an aromatic source because of its chemical structure. In addition lignin results by the cellulose production in large quantities.
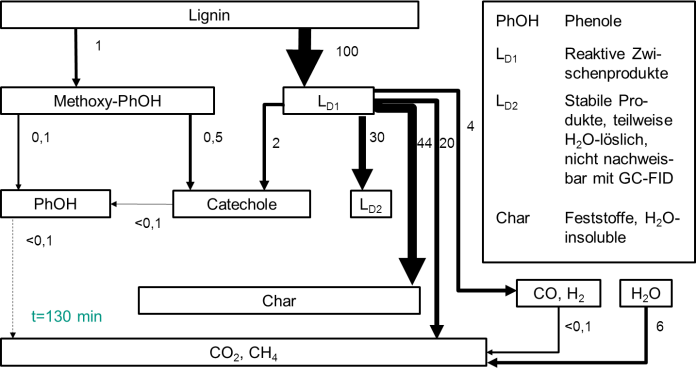
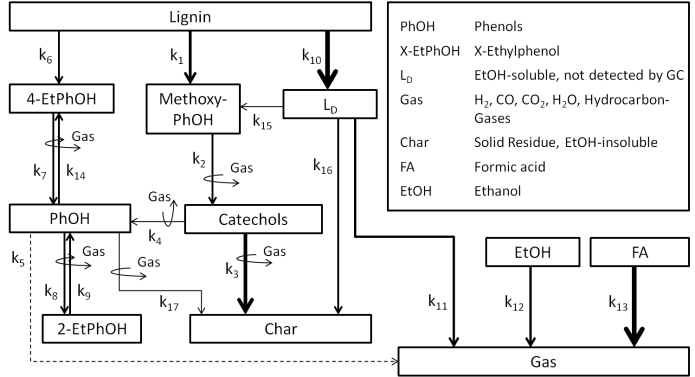
Actually the degradation and conversion of the lignin lead to monophenolic substances like catechol and phenol which are interesting basic chemicals. A challenge referring to the syntheses are the rather lower yields of these products. In order to get better yields it's very important to understand the whole reaction path ways starting from the formation of the products to the following subsequent reactions. Because of this background reaction models were created for the conversion of lignin in water and ethanol in the close-critical range under special consideration of monophenolic substances. Both models show many parallels and some solvent specific reactions were adapted. They describe the conversion of lignin at different temperatures as well as in different reactors due to reaction volume and operating methods. A comparison of the sensitivity analyses shows that the formation of phenol runs in water preferentially through methoxyphenoles while in ethanol the reaction way about oligomeres is preferred. The developed models were validated in different reactors (batch reactor, continuous plug flow reactor and continuous stirring reactor) with a reaction volume of 10 ml to 350 ml.
The aim of these works is to create a tool in order to make predictions for innovative processing concepts. This can give us the possibility to develop new and efficient syntheses of platform chemicals like catechol and phenol from the lignin.
Literature
- Gasson, J.R.; Forchheim, D.; Sutter, T.; Hornung, U.; Kruse, A.; Barth, T.;
Modeling the lignin degradation kinetics in an ethanol / formic acid solvolysis approach - Part I: Kinetic model development,
Ind. Eng. Chem. Res., 51(2012)10595-10606, DOI:10.1021/ie301487v. - Forchheim, D.; Gasson, J.R.; Hornung, U.; Kruse, A.; Barth, T.;
Modeling the lignin degradation kinetics in an ethanol / formic acid solvolysis approach - Part II: Validation and transfer to variable conditions,
Ind. Eng. Chem. Res., 51(2012) 15053-15063, DOI:10.1021/ie3026407. - Forchheim, D.; Hornung, U.; Kempe, P.; Kruse, A.; Steinbach, D.:
Influence of RANEY-Nickel on the formation of intermediates in the degradation of lignin,
International Journal of Chemical Engineering, 2012, doi:10.1155/2012/589749. - Forchheim, D.; Hornung,U; Kruse,A.; Sutter, T.;
Kinetic modelling of hydrothermal lignin depolymerisation, Waste and Biomass Valorization,
submitted.