Institute of Catalysis Research and Technology (IKFT)
The Institute of Catalysis Research and Technology was founded 2011. Its mission is to bridge the gap between fundamental and applied research and the development of new technologies and products in the field of catalysis and process technology of catalyzed processes. The focus of our work is the sustainable utilization of alternative feedstocks and their conversion into energy carriers intermediates. This includes the development of new catalytic systems based on a fundamental understanding of processes on a molecular level. The institute receives basic funding from the Helmholtz Association's program-oriented funding, largely in the Energy research field in the program Materials and Technologies for the Energy Transition.
_rdax_98s.jpg)
On 21 November, the Night of Science took place at KIT, and IKFT contributed to the vibrant program.
mehr
The DGMK event Hydrogen and Syngas - Platform for a sustainable future was jointly organized by the DGMK Divisions "Petrochemistry" and "Conversion of Carbon Carriers", the Division of Industrial Chemistry of the Società Chimica Italiana (SCI) and the ÖGEW Österreichische Gesellschaft für Energiewissenschaften.
more
The NFDI4Cat 2.0 kick off meeting took place on 22 and 23 October 2025 at the DECHEMA Haus in Frankfurt (Main). In this second funding period, TT-Prof. Dr. Moritz Wolf joins as a Participant and introduced his activities in data-driven research to the consortium. Together with the established principal investigator Prof. Dr. Olaf Deutschmann and Dr. Sofia Angeli IKFT will shape the future of catalysis-related data and research.

A large group of researchers from KIT attended the 16th European Congress on Catalysis (EuropaCat 2025) in Trondheim, Norway (31.08 to 05.09.2025). Professors, postdoctoral and doctoral researchers from IKFT, as well as ITCP and ITC, contributed with posters, oral presentations, and a plenary lecture, showcasing the catalysis research at KIT.
more
Liana Savintseva won the ETOS Research Award. Congratulations!
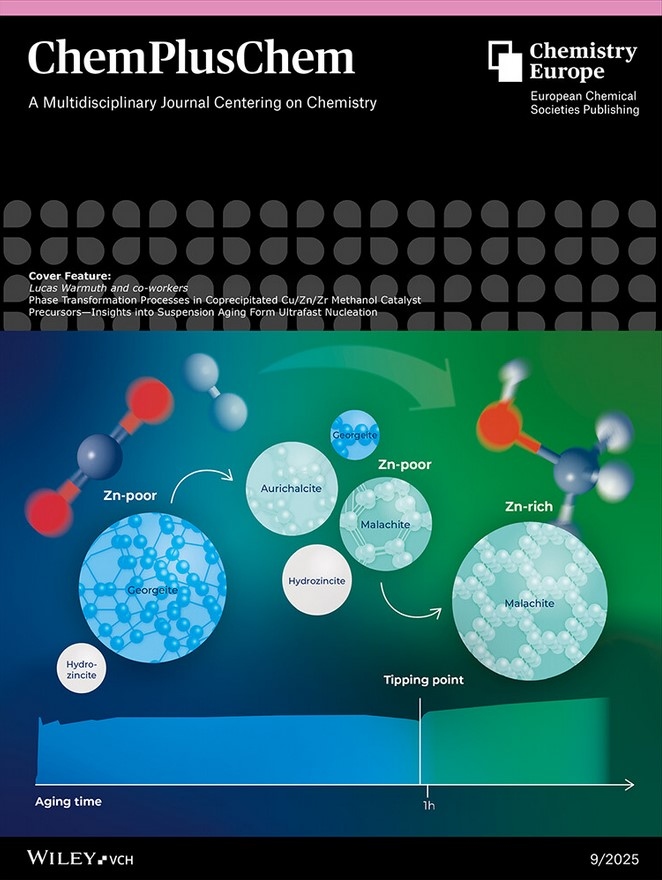
Suspension aging is critical in the synthesis of Cu/Zn-based methanol catalysts, because this process of chemical transformations includes crystallization of different phases. The evolution of these phases within the precipitate is leading along the so-called transitory tipping point to the target phase zincian malachite. More information can be found in the Research Article by Lucas Warmuth and co-workers (DOI: 10.1002/cplu.202500284).
Veranstaltungskalender
Non-Nernstian effects and kinetics-informed descriptors in electrocatalysis
Non-Nernstian effects and kinetics-informed descriptors in electrocatalysis
Prof. Dr. Georg Kastlunger,
Technical University of Denmark
Fysikvej, Kongens Lyngby, Denmark
geokast ∂does-not-exist.dtu dk
Thermodynamic descriptors are among the most central tools to predict electrocatalytic activity. They are mostly founded on the analysis of reaction thermodynamics or empirical correlations with experiments, often masking the influence of the kinetics and reaction conditions at play. However, a deep atomistic understanding of complex reaction mechanisms in electrocatalysis aids not only the discovery of improved catalytic materials but also the choice of ideal reaction environments for tailored products.
In my talk, I will present density functional theory-based studies on electrocatalytic reaction mechanisms with a special focus on water splitting, electrochemical CO(2) reduction and biomass electrovalorization. I will discuss how non-Nernstian effects influence electrocatalytic observables1 and their essential role in rationalizing improved descriptors from first principle kinetics even for simple reactions such as hydrogen evolution.2 I will describe how these electrochemical descriptors are essential to resolve the potential, pH and electrolyte dependence of multistep reaction networks,3 highlighting that a descriptor-based search for novel catalysts can be drastically refined if the mechanisms and relevant driving forces are analyzed appropriately.
1. Tripathi, A., Ocampo-Restrepo, V. K., Nørskov, J. & Kastlunger, G. Field effects explain the unintuitive potential response of electrochemical oxygen evolution in acid. RSC Sustainability 3, 2659–2668 (2025).
2. Patel, D. M., Ghan, S., Vishart, A. L. & Kastlunger, G. Improved hydrogen evolution activity descriptors from first-principles electrochemical kinetics. Electrochim Acta 543, 147476 (2025).
3. Kastlunger, G., Heenen, H. H. & Govindarajan, N. Combining First-Principles Kinetics and Experimental Data to Establish Guidelines for Product Selectivity in Electrochemical CO 2 Reduction. ACS Catal 13, 5062–5072 (2023).
Prof. Dr. Georg Kastlunger
