Institute of Catalysis Research and Technology (IKFT)
The Institute of Catalysis Research and Technology was founded 2011. Its mission is to bridge the gap between fundamental and applied research and the development of new technologies and products in the field of catalysis and process technology of catalyzed processes. The focus of our work is the sustainable utilization of alternative feedstocks and their conversion into energy carriers intermediates. This includes the development of new catalytic systems based on a fundamental understanding of processes on a molecular level. The institute receives basic funding from the Helmholtz Association's program-oriented funding, largely in the Energy research field in the program Materials and Technologies for the Energy Transition.

The DGMK event Hydrogen and Syngas - Platform for a sustainable future was jointly organized by the DGMK Divisions "Petrochemistry" and "Conversion of Carbon Carriers", the Division of Industrial Chemistry of the Società Chimica Italiana (SCI) and the ÖGEW Österreichische Gesellschaft für Energiewissenschaften.
more
The NFDI4Cat 2.0 kick off meeting took place on 22 and 23 October 2025 at the DECHEMA Haus in Frankfurt (Main). In this second funding period, TT-Prof. Dr. Moritz Wolf joins as a Participant and introduced his activities in data-driven research to the consortium. Together with the established principal investigator Prof. Dr. Olaf Deutschmann and Dr. Sofia Angeli IKFT will shape the future of catalysis-related data and research.

A large group of researchers from KIT attended the 16th European Congress on Catalysis (EuropaCat 2025) in Trondheim, Norway (31.08 to 05.09.2025). Professors, postdoctoral and doctoral researchers from IKFT, as well as ITCP and ITC, contributed with posters, oral presentations, and a plenary lecture, showcasing the catalysis research at KIT.
more
Liana Savintseva won the ETOS Research Award. Congratulations!
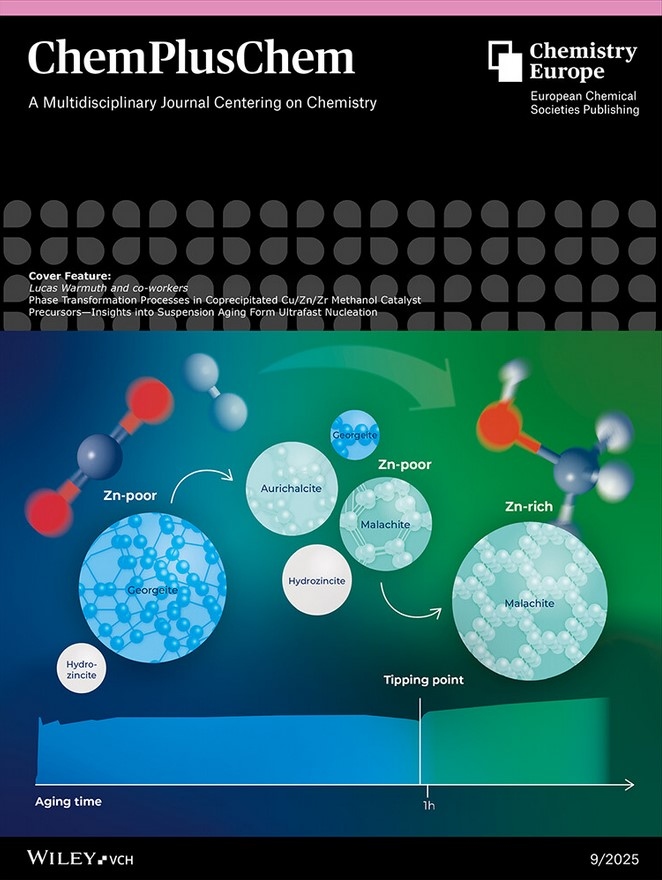
Suspension aging is critical in the synthesis of Cu/Zn-based methanol catalysts, because this process of chemical transformations includes crystallization of different phases. The evolution of these phases within the precipitate is leading along the so-called transitory tipping point to the target phase zincian malachite. More information can be found in the Research Article by Lucas Warmuth and co-workers (DOI: 10.1002/cplu.202500284).

A recent investigation of the particle shape of cobalt-based Fischer–Tropsch catalysts using Monte Carlo simulations was featured as a front cover in the Journal of Physical Chemistry C. J. Phys. Chem. C 2025,129,13232−13243. https://doi.org/10.1021/acs.jpcc.5c02777
Veranstaltungskalender
Cation Effects on the Acidic Oxygen Reduction Reaction at Carbon Surfaces IKFT & HyPerCat Seminar
Cation Effects on the Acidic Oxygen Reduction Reaction at Carbon Surfaces
Jessica Hübner, Technical University Berlin, Straße des 17. Juni 124, 10623 Berlin
Hydrogen peroxide (H₂O₂), a green oxidant widely used in industry, can be generated through the two-electron oxygen reduction reaction (2e⁻ ORR). Alkaline 2e⁻ ORR is already applied at scale, but acidic H₂O₂ solutions are often preferred. Under low pH, however, the 2e⁻ ORR typically shows lower activity and selectivity. This limitation can be addressed by adjusting the electrolyte composition.
Using commercial glassy carbon electrodes, the effect of alkali metal cations (AMCs) in the electrolyte, exemplified by K⁺, on the acidic 2e⁻ ORR was examined. Rotating ring-disk electrode (RRDE) experiments and in situ X-ray photoelectron spectroscopy (XPS) revealed a strong potential-dependent activity enhancement. Density functional theory calculations indicate that the activity enhancement arises from stabilization of the *OOH key intermediate.
Building on these insights, a flow cell utilizing commercial carbon gas diffusion electrodes and optimized electrolytes containing different mono- and multivalent cations achieved high H₂O₂ production rates with close to 100% Faradaic efficiency. These results demonstrate that tuning the electrode–electrolyte interface is a key strategy for efficient, scalable, and decentralized H₂O₂ electrosynthesis.
Dr. Jessica Hübner
Technical University Berlin
